Equipment
Transmission electron microscopy (TEM) allows visualisation of morphology and structural details of biological and material samples such as: organelles, proteins, cells membranes, polymers, vesicles, exosomes, nanoparticles, semiconductors, crystalline structure. TEM uses a transmitted electron beam that passes through the specimen to form an image. The specimen must be less than 100 nm thick and specially prepared. The resolution that can be reach with this microscope is around 0.3nm.

Joel JEM -1400 Plus Transmission Electron Microscope
- Acceleration voltage 40kV to 120kV
- Magnification x10 to x800,000
- JEOL Ruby 8MP Bottom mounted CCD digital Camera
- Standard specimen holder
- +/- 70º tilt with support for tomography
- High Tilt tomography holder
- Liquid Nitrogen Cold Trap -Cryo ready
- 2D montaging and 3D tomography
- Selected area electron diffraction
Related links
Automated tissue processor Leica EM-TP
Allows automatic biological sample preparation for transmission electron microscopy. It has a heating/cooling system that maintain constant processing temperature for over 24h. The carousel holds 24 vials and utilizes specialized baskets and capsules that allows exchange of buffers. An exhaust system supports safer use of toxic substances.
Related Links:
LEICA Ultracut - UCT ultra-microtome
Uses a glass or diamond knife to cut semi and ultrathin sections to prepare electron microscopy samples. Sections can be between 70-100 nm thick and 0.5 to 2mm wide and can be stained and mounted on the TEM grids for visualization.
Related Links:
Widefield fluorescence microscopy is an optical technique where the sample is illuminated with visible light of a specific wavelength to excite fluorophores attached to different molecules in cultured cells or tissues. This technique can be used to identify cells, cell membranes, organelles, proteins, lipids in live or fixed samples.

INSTRUMENTS
Leica DM5500B Upright Microscope
Can be used for fixed samples only.
Allow examination of histopathological samples with Leica DFC 290 colour digital camera.
Bright field and fluorescence images can be recorded using Leica DFC340 monochrome digital camera.
Objectives :
- 2.5x/ 0.07 NA PL FLUOTAR
- 5x/ 0.15 NA HCX PL FLUOTAR
- 10x/ 0.3 NA HCPL FLUOTAR Ph1
- 20x/ 0.5 NA PL FLUOTAR-Ph2
- 40x/ 0.85 NA HCX PL APO, CORR
- 100x/ 1.4-0.7NA HCX PLAN APO oil
Filter cubes
1.Excitation: 360nm±40;Emission: 470nm±40
2.Excitation: 480nm±40;Emission: 527nm±30
3.Excitation: 560nm±40;Emission:645nm±75
4.Excitation: 620nm±60;Emission:700nm±75
Related Links:
Nikon AZ100 Multi Zoom upright microscope
It is a compound microscope designed to visualize large specimens in epifluorescence or DIC (Differential Interference Contrast) methods. With low magnifying, long working distance objectives and a zooming mechanisms it digitally magnify between 5x to 400x.
Histopathological samples can be visulaized too due to the color camera.
Objectives:
- AZ Plan Apo 1x NA 0.1 (working distance 35mm)
- AZ Plan Fluor 2x NA 0.2 (working distance 45mm)
- AZ Plan Fluor 5x NA 0.5 (working distance 15mm)
By an optical zoom mechanism each objective's magnification can be increased up to x 8 resulting in 40x (with objective 5x and zoom-factor 8x) magnification.
Filter cubes:
1.Excitation:340nm-380nm;Emission:435nm-485 nm
2.Excitation:465nm-495nm;Emission:515nm-555 nm
3.Excitation:540nm±25nm ;Emission:605nm±55nm
Camera:
Nikon DS-Ri1 colour camera. Two resolution settings are possible: 1636 x 1088 pixel or 4908 x 3264 pixel.
Related Links:
Nikon 6D Live Cell Imaging inverted Microscope
It is an automatic system that allow multi-dimensional time-lapse imaging of live or fixed cultured cells in multi-well plates, slide or petri dish within a controlled environment. Tile scanning on the fixed or live samples is possible.
Perfect Focus System (PFS) corrects focus drift in real time during the multi point acquisition.
Objectives:
- 10x / 0.13 NA CFI60 Plan Fluor DLL
- 20x / 0.75 NA CFI60 Plan Apochromat VC
- 40x / 0.6 NA CFI60 Super Plan Fluor ELWD
- 40x / 1.3 NA CFI60 Plan FLUOR oil
- 60x / 1.4 NA CFI60 Apochromat Lambda S oil
- 100x/ 1.45 NA CFI60 Plan Apochromat Lambda oil
Filter cubes:
1. Excitation: 405 nm; 470 nm; 630nm
2. Excitation: 385 nm; 505 nm; 525 nm; 560 nm; 625nm
3. FITC
4. Texas Red
Camera:
Neo 5.5 sCMOS ANDOR Camera with 5.5Megapixels and 2560x2160 16.6 mm x 14.0 mm sensor size.
Light source:
Lumencor Spectra X- LED Light Engine with six solid-state light sources that generate seven colour bands (violet, blue, cyan, teal, green, yellow and red).
Related Links:
EVOS- FL AUTO 2 CELL IMAGING SYSTEM
EVOS is an automated inverted four-channel fluorescence and transmitted light imaging system that can be used for imaging fixed or live biological samples in well plate or fluorescence slides. The colour camera allows imaging of fixed histopathology samples.
Time lapse images can be recorded for live cells with the environmental control.
EVOS FL Auto 2 system can be programmed to run well-plate (plastic or glass) scanning, time lapse experiments, and tile-stitch/montage area scans—in Z-stack and/or time-lapse modes.
Objectives:
- 4x / 0.13 NA AMG LPlan FL PH
- 10x/ 0.25 NA AMG LPlan FL PH
- 20x/ 0.4 NA AMG LPlan FL PH
- 40x/ 0.65 NA AMG LPlan FL PH
- 40x / 0.75 NA Plan FL Cover Slip
Filter cubes:
1. DAPI:Excitation: 360nm; Emission: 447 nm
2. GFP : Excitation: 470 nm;Emission: 535nm
3. Texas Red: Excitation: 530 nm;Emission: 593nm
4. CY5: Excitation: 628 nm;Emission: 692nm
Camera:
-1.3 MP CMOS monochrome camera with 1328x1048 pixels
-High sensitivity 3.2 MP CMOS color camera with 2080x1552 pixels
Related Links:
Confocal microscopy is an optical technique that eliminates out of focus light by adding a pinhole in the light path. Laser scanning confocal microscopy produces thin (0.1 to 1.5 micrometre) optical sections through fluorescent specimens that have a thickness of 100 micrometres or more.
Confocal microscopes can be used to visualize the structure of cells, drug distribution in the cells, organelles, cell membranes protein distribution or interactions. Using the FRET (Forster Resonance Energy Transfer) or FRAP (Fluorescence Recovery After Photo bleaching) module it is possible to detect if two molecules are within 10 nm of each other or to investigate the diffusion or movement of the molecules in a specific area of the cells or tissue.

Instruments
Leica TCS-SP5 inverted microscope
This can be used for fixed or live samples. It uses a spectral detection, that mean all dyes that can be excited with the availble lasers can be detected.
Lasers
- 405 nm Blue diode laser
- Argon laser with excitation wavelengths 458 nm, 476 nm, 488 nm, 496 nm and 514 nm
- 561 nm DPSS-Diode
- 594 nm Helium Neon Gas laser
- 633 nm Helium Neon Gas laser
Objectives:
- 20x / 0.5NA HC PL Fluotar
- 20x / 0.7NA HCXPL APO Lambda blue IMM UV
- 40x / 0.75 NA HCXPL Fluotar
- 40x / 1.25 NA HCX PL APO, PH3 -oli
- 63x / 1.4 NA HCX PLAPO Lambda blue- oil
- 100x/ 1.4NA HCX PL APO -oil
Software for image acqusition : LAS AF
Leica confocal microscopy webinar
Related Links:
Leica TCS SP8 inverted microscope
Leica TCS SP8 confocal has been designed with optimal photon efficiency and high speed. This system has 4 detectors (2 sensitive Hybrid GaAsP and 2 PMT) and a motorized xyz-stage.
Time lapse imaging of live cultured cells or tissue is possible due to the controlled environment.
Because of the spectral detection any dyes that can be excited with the avaiable lasers can be detected.
Resonant scanner for fast image aqusision is available.
Objectives:
- 10x / 0.4 NA HC PL FLUOTAR
- 20x / 0.75 NA HC PL APO CS2
- 40x / 1.10 NA HC PL APO W CORR CS2 water
- 40x/ 1.25 NA HCX PL APO PH3 oil
- 63x / 1.4 NA HCX PL APO lambda blue oil
- 100x/ 1.4 NA HC PL APO CS2 oil
Laser lines for excitation:
- 405 nm
- 488nm
- 510 nm
- 552nm
- 638nm
Related Links:
LAS X software for image processing
Spectral detection with Leica SP8
Leica STELLARIS 5 inverted microscope
Lasers lines for excitation:
- 405 nm
- 488 nm
- 555 nm
- 638nm
Objective:
- 10x / 0.4 NA HC PL APO CS2
- 20x / 0.75 NA HC PL APO CS2
- 40x / 1.3 NA HC PL APO CS2 oil
- 63x / 1.4NA HC PL APO CS2 oil
- 100x / 1.4 NA HC PL APO CS2 oil
Related Links:
LAS X software
Leica SP8- upright microscope
This microscope is designed mainly for living samples. It can be used for fixed samples too and immunofluorescence detection.
Spectral detection via 2 PMT’s and 2 sensitive Leica Hybrid detectors.
Resonanat scanner for fast image acqusition is available.
Objectives
- 10x / 0.4NA HC PL APO, PH1
- 10x / 0.3NA HCX APO L W. water immersion
- 20x / 0.5NA HC PL FLUOTAR
- 25x / 0.95NA HC FLUOTAR W VISIR; High transmission >83% from 400nm to 1300nm. Colour corrected for VIS and NIR up to 950nm. water immersion
- 40x / 0.6 NA HCX PL FL L CORR PH2 02/C
- 40x / 0.85NA HCX PL APO CORR CS 0.11 superior colour correction
- 63x / 1.2 NA HC PL APO W CORR CS2; water immersion
- 63x / 0.9 NA HC APO L W UV1 CS2 water immersion
Lasers
- 405 nm diode laser
- 488 nm diode laser
- 514 nm diode laser
- 552 nm diode laser
- 638 nm diode laser
Related Links:
LAS X software for image acqusition and analysis
Many cellular events like viral infection, endocytosis, receptor-ligand interaction, cytoskeletal dynamics, movement of organelles in the vicinity of the membranes, take place at the cell surface; with TIRF those events can be visualized with low noise or interfernce from near regions. Changes in the orientaion of the molecules can be detected with the polarization controled electromagneric field orientation.
Total internal reflection fluorescence (TIRF) or evanescent wave microscopy is a technique where fluorophores are illuminted by an electromagnetic field that is generated by the total internal reflection of the incident laser beam at the interface of media with different index of refraction like water and glass. Electromagnetic field restricted to 100nm-150nm deep into the specimen can excite individual molecules with low background fluorescence , low light exposure and no out of focus fluorescence.

Leica TIRF microscope uses objective to generate the evanescent electromagnetic field.
Excitation lasers:
- 405 nm
- 488 nm
- 561nm
- 632 nm
Objectives:
- 10x / 0.4NA HC PL APO
- 20x / 0.7NA HC PL APO
- 40X/ 0.85NA HC PL APO
- 63x / 1.47NA HC PL APO Optimized for TIRF oil immersion
- 100x / 1.47NA HC PL APO Optomized for TIRF oil immersion
Filter cubes:
1.QUAD cube for TIRF fast acqusition:
Excitation : 405±10nm ; 488±13 nm; 561±10nm; 635±15nm
Emission : 450 ± 55nm ; 525±50nm ; 605±45nm; 730±100nm
2. CFP Excitation : 436±20nm ; Emission 480±40nm
3. GFP Excitation : 470±40nm ; Emission 500±40nm
4. CY3 Excitation : 545±25nm ; Emission 595±50nm
5. CY5 Excitation : 640±30nm ; Emission 690±50nm
Camera:
-Andor Zyla 4.2 sCMOS Camera with 4.2 Megapixel, 82% QE
Links:
Two-photon excitation microscopy (TPEM) is a nonlinear optical technique where fluorescent dyes are excited by absorbing the energy of two photons simultaneously at double the wavelength used in confocal microscopy. To excite fluorophores, multiphoton microscopy uses infrared pulsed laser beam that has lower energy than the visible light used in 1 photon microscopy. Because of this, less light scatter so fluorophores in thicker tissue (1mm) can be detected and long-time imaging can be acquire because of less photo-bleaching and photo-destruction.
FLIM- Fluorescence lifetime imaging microscopy module is attached to the Leica TCS-MP microscope.

Excitation source:
Multi-photon laser: Mai Tai eHP DeepSee IR laser excitation from 690-1040nm, pre-chirped/short pulse width for compensation with deep tissue imaging- high performance / low scattering.
Objectives:
- 10x/ 0.4NA HC PL APO, PH1
- 10x/0.3NA HCX APO L W
- 20x/ 0.5NA HC PL FLUOTAR
- 25x / 0.95 NA HC FLUOTAR L W VISIR , High transmission >83% from 400nm-1300nm. Colour corrected for VIS and NIR up to 950nm- water immersion
- 40x /0.6NA HCX PL FL L CORR PH2
- 40x / 0.85NA HCX PL APO CORR CS 0.11 superior colour correction
- 63x / 1.2NA. HC PL APO W CORR CS2 water immersion
- 63x/ 0.9NA HC APO L W UVI CS2 water immersion
Techniques
- Fluorescence imaging
- Spectral imaging
- Fluorescence lifetime imaging with Pico Quant system
Time Correlated Single Photon Counting (TCSPC) measures the fluorescence decay of the fluorophores. Fluorescence lifetime is an endogenous properties of the fluorophore defined as the average time that molecule remains in an excited state before deactivate and return to the ground state by emitting a photon. The image that creates it is based on the differences in the excited state decay rate (fluorescence lifetime) of the fluorophores.
Fluorescence lifetime does not depend on concentration, absorption by the sample, sample thickness, photo-bleaching and/or excitation intensity . At the same time, the fluorescence lifetime depends on a wealth of environmental parameters such as pH, ion or oxygen concentration, molecular binding or the proximity of energy acceptors making it the technique of choice for functional imaging of many kinds.
Related Links:
The system includes
- Mai Tai eHP DeepSee IR laser tunable from 690nm to 1040 nm pre-chirped/short pulse width for compensation with deep tissue imaging- high performance / low scattering.
- Resonant module scanner that allow acquisition of 30 fps @ 512x512.
- 4 channel simultaneous acquisition using: 2 sensitive GaAsP, hybrid and two PMT detectors.
- 2-Channel TCSPC Detector
- Automated stage for multi-position imaging
- Incubation system for temperature, humidity and CO2 control
STED system allow visualization of biological structure , nanocomposites , semiconductors at 30 nm resolution
The system is based on a confocal microscope equipped with a pulsed White Light Laser for excitation and with a 660 nm laser for depletion. It can be used for immunofluorescence , FRET, FRAP analysis.
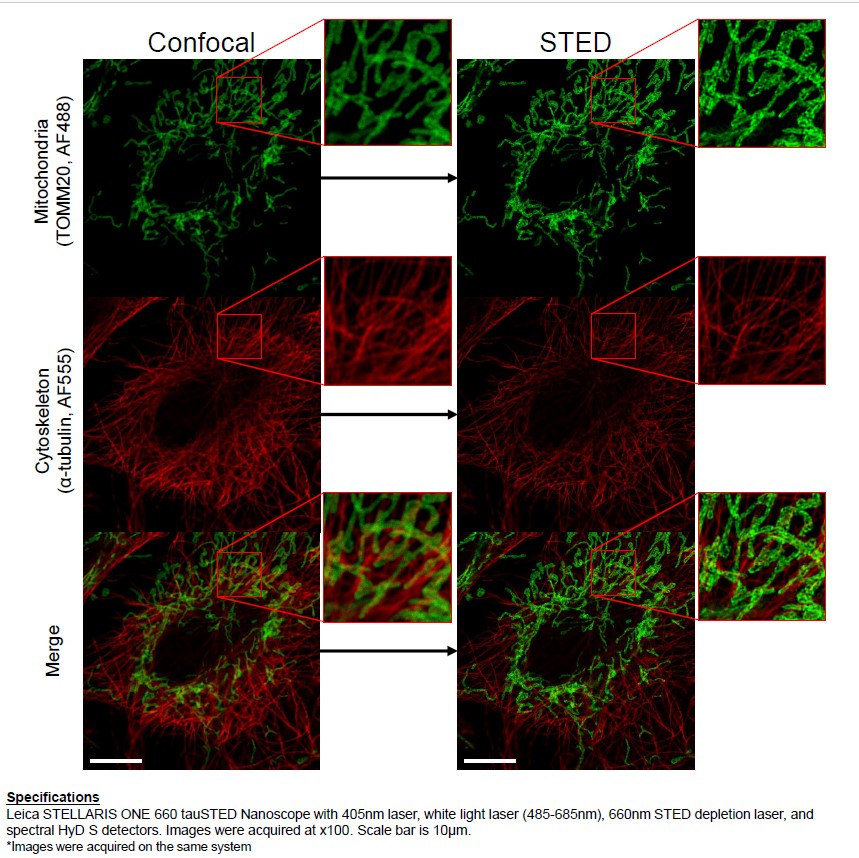
Capabilities:
-White Light laser with pulsed excitaion from 485 nm to 68 nm tunable in steps of 1nm with an AOTF (Acousto Optical Tunable Filter) for rapid modulation of the laser intensity
-tauSENSE is a module based on lifetime imaging that allow separation of the spectrally overlapping fluorophores, provides additional contrast and improve the quality of the image
-Imaging fluorophores with excitaion from 400 nm to 685 nm and emission between 405 to 850 nm using spectral detection.
-Brightfield detector with PMT detector
-Allows tauSense functionality: tauGating, tauContrast, tauSepartaion, tauScan
-4 internal detection channels -4 Power HyDS, photon counting hybrid detector with spectral sensitivity range : 410 to 850 nm. The Power HyD S is based on silicon multi-pixel photon counter (MPPC) technology
-Active CO2 and temperature controller
-Software: LAS X for imaging and analysis
The Navigator software that can image the full sample by creating an overview of the sample using spiral scan function and acquire a mosaic scan of the sample and find regions of interest rapidly.
Objectives:
-20x 0.75 NA , HC PLAPO CS2 , AIR, Working distance 0.62 mm
-40x 1.3 NA, HC PL APO CS2, OIL, Working distance 0.24 mm
-63x 1.4 NA, HC PL APO CS2, OIL, Working distance 0.14 mm
-100x1.4 HC PL APO CS2, OIL, Working distance 0.13 -optimized for STED
The Molecular Devices FlexStation 3 is a benchtop scanning fluorometer and integrated fluid transfer workstation capable of conducting endpoint, kinetic, absorbion , emission spectrum scan,l uminescence and well scan experiments in a multi-well plate format (6, 12, 24, 48 and 96-well) and fluidics transfer experiments in 96-well format. The FlexStation 3 is ideal for measuring the activity of receptors by quantifying intracellular calcium release. In addition, the measurement of enzyme activity, GMP and AMP levels, potassium ion channel flux, and membrane potential is possible.
This multi-mode plate reader with 8 integrated liquid dispensers supports the following applications:
Automated system allowing rapid and high throughput screening of a large panel of biological parameters
- Absorbance (200nm-1000nm)
- Fluorescence (250 nm- 850 nm)
- Luminescence (250-850 nm)
- Dual monochromators (1nm)